FT522
FT522 is our investigational, off-the-shelf, iPSC-derived CAR NK cell product candidate, and our first product candidate to incorporate our novel alloimmune defense receptor (ADR) technology which is designed to reduce or eliminate the need for administration of conditioning chemotherapy to patients receiving cellular immunotherapy. While approved autologous CAR T-cell therapies have demonstrated compelling efficacy in treating patients with relapsed / refractory hematologic malignancies, several key challenges limit its adoption and patient reach including the need to co-administer conditioning chemotherapy to patients. Conditioning chemotherapy induces toxicities, necessitates administration in large hospitals and treatment centers with intensive care units, and prevents effective combination with standard-of-care treatment regimens widely used in the community setting.
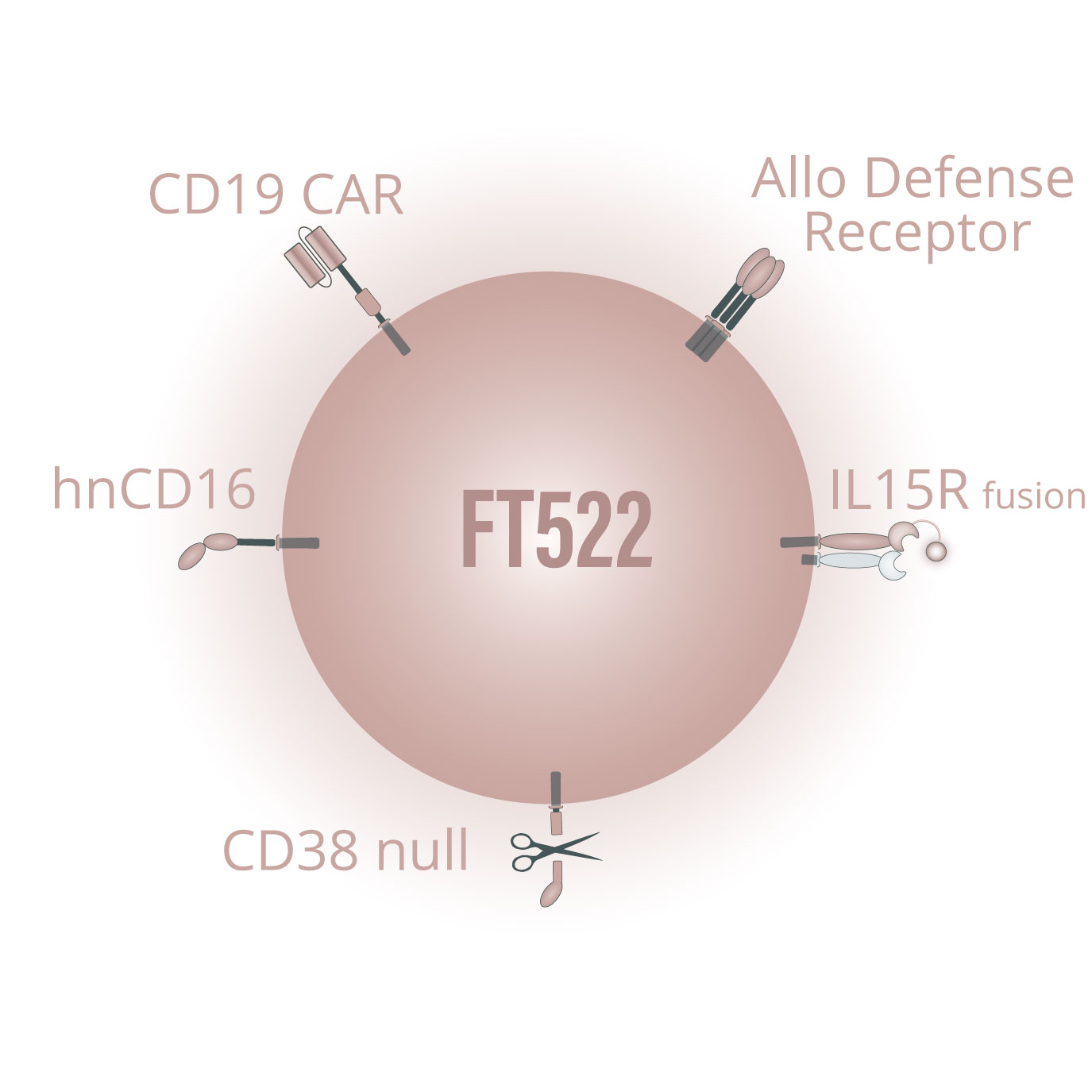
The ADR receptor is designed to (a) selectively recognize and destroy alloreactive host immune cells that would otherwise be capable of rejecting the product candidate, (b) maintain other components of the host immune system to preserve hematopoietic cell function, and (c) activate the product candidate to enhance its potency and persistence. Our preclinical data suggest that FT522 has the potential to robustly deplete CD19+ B cells, evade host immune cell rejection, and drive clinical responses without administration of intense conditioning chemotherapy to patients. We are currently evaluating opportunities and timelines for the clinical development of FT522.
We are evaluating opportunities for the clinical development of FT522 in autoimmune diseases. In October 2024, the FDA allowed our Investigational New Drug (IND) application to assess the safety, pharmacokinetics, and activity of FT522 across a basket of B cell-mediated autoimmune diseases including anti-neutrophilic cytoplasmic AAV, IMM, SSc, and SLE. The Phase 1 clinical protocol permits treatment of patients with up to four weekly doses of FT522, without administration of conditioning chemotherapy, as an add-on to rituximab induction therapy (Regimen A) and as an add-on to maintenance therapy in combination with rituximab (Regimen B).